Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that is associated with long-term disability and increased mortality.1,2 The articular inflammation and joint destruction in RA are often accompanied by systemic manifestations, including anemia, fatigue, osteoporosis, and cardiovascular disease.1,2 The worldwide prevalence of RA is approximately 1%, with women disproportionately affected; the common age of disease onset is between 35 and 50 years.1,2
The current treatment armamentarium for RA features several agents with varying mechanisms of action, including conventional synthetic disease-modifying antirheumatic drugs (DMARDs), targeted synthetic DMARDs, and biologic DMARDs, that are directed toward ameliorating symptoms and modifying the disease process.3 The conventional DMARD methotrexate continues to be the cornerstone of DMARD therapy; however, up to 50% of patients discontinue methotrexate at 1 year, and adverse effects can occur from its prolonged use.4
An increased understanding of the molecular pathogenesis of RA has led to significant advances in biologic therapies in the past 15 years and has dramatically changed the treatment paradigm for RA.3 Currently, 4 classes of biologic DMARDs are available, including (1) the tumor necrosis factor (TNF)-α inhibitors adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab; (2) abatacept, which blocks T-cell costimulation via anti-CD80/86 inhibition; (3) the anti-CD20 agent that causes B-cell depletion, rituximab; and (4) the interleukin (IL)-6 receptor (IL-6R) blocker, tocilizumab. These agents are indicated for patients with an inadequate response to conventional DMARDs.3
TNF inhibitors are regarded as the standard of care in patients who have an inadequate response to methotrexate; however, up to 40% of patients with RA have persistent disease activity even after therapy with TNF inhibitors, and the use of TNF inhibitors is contraindicated in certain patient populations.5 Because only 5% to 10% of patients with RA achieve and maintain remission despite the currently available treatment options, new and effective therapies are needed to address the unmet needs of patients with moderate-to-severe RA activity.5
Sarilumab is a novel IL-6R inhibitor that has demonstrated encouraging activity in moderate-to-severe RA. This monograph describes the rationale for anti–IL-6 strategies and the clinical evidence-base for sarilumab within the context of the current (2015) American College of Rheumatology (ACR) guidelines for RA treatment.6
The Current ACR Guidelines Recommendation for the Use of Biologic DMARDs
Given the number of available biologic treatments for RA, the ACR has assessed the level of evidence in the literature that supports current treatment options for patients with RA, based on the benefits, risks, and patient preferences for various biologic DMARDs, and proposed recommendations to guide providers in developing treatment plans.6
Because RA can have debilitating effects and can substantially reduce patients’ quality of life, an early diagnosis and prompt treatment is desirable to achieve a therapeutic response.6 In early (<6 months disease duration) and established (≥6 months disease duration) RA disease settings, the ACR panel advocates using a treat-to-target strategy over a nontargeted treatment approach, regardless of the level of disease activity, but with low disease activity or remission being the ideal treatment target.6
For DMARD-naïve patients with early or established symptomatic RA and moderate-to-high disease activity, the ACR guidelines recommend monotherapy with conventional DMARDs, preferably methotrexate, as initial therapy.6 This is a notable change from the 2012 ACR guidelines; the current ACR guidelines do not recommend combination DMARD therapy as first-line treatment in patients with early RA and moderate-to-high disease activity.6
In the second-line setting, after failure with conventional DMARDs, patients with established RA may receive a combination of conventional DMARDs or add a TNF inhibitor or a non-TNF inhibitor biologic drug or tofacitinib (all choices, with or without methotrexate, and in no particular order of preference), rather than continuing conventional DMARD monotherapy alone.6 Whenever possible, biologic therapy should be used in combination with methotrexate, because of the combination’s superior efficacy over biologic monotherapy.6
If active disease persists despite single TNF inhibitor biologic therapy, switching to a non-TNF biologic drug, with or without methotrexate, is recommended over another TNF inhibitor or tofacitinib. Switching to a biologic drug with a novel mechanism of action rather than using another TNF inhibitor is a notable new recommendation; this recommendation applies even in the case of persistent disease activity despite single non-TNF inhibitor therapy, or at least 1 TNF inhibitor and at least 1 non-TNF biologic drug (sequentially, not combined), or multiple (≥2) sequential TNF inhibitor therapies.6
If disease activity is low, the ACR guidelines recommend continuing therapy with DMARDs, TNF inhibitors, non-TNF biologic therapy, or tofacitinib, rather than discontinuing therapy.6 Treatment should continue even if RA is in remission; tapering of therapy to lower doses is recommended once low disease activity or clinical remission is reached to prevent flare-ups and the deterioration of health status.6
Treatment Considerations in Special Patient Populations
The ACR guidelines recommend personalizing treatment plans for patients with RA, based on their medical history and the presence of high-risk comorbidities, including cardiovascular disease, malignancy, and infections.6 The US Food and Drug Administration (FDA) cautions against the use of TNF inhibitors in patients with congestive heart failure (CHF), because TNF inhibitors are associated with worsening of CHF. Therefore, the guidelines recommend using a combination of DMARDs, a non-TNF inhibitor biologic drug, or tofacitinib, over TNF inhibitors in these patients.6
The ACR panel considered the risk for recurrence of malignancies with the use of biologic DMARDs in patients with a history of melanoma or nonmelanoma skin cancer or lymphoproliferative disorders.6 For melanoma or nonmelanoma skin cancer, conventional DMARDs are conditionally recommended over biologic drugs or tofacitinib, because conventional DMARDs are associated with a reduced risk for recurrence of malignancies.6
For patients with a history of lymphoproliferative disorders, the panel strongly recommends using rituximab over a TNF inhibitor, and conditionally recommends using a combination of DMARDs or abatacept or tocilizumab over TNF inhibitors, based on evidence that TNF inhibitors may increase the risk for lymphoma in these patients; no such evidence exists for abatacept or tocilizumab.6 For patients with RA who have received treatment for solid malignancies, the same treatment recommendations apply as for patients without a history of malignancies.6
For patients with RA and hepatitis B or C infection, the ACR guidelines suggest that immunosuppressive therapy can be safely used when prophylactic antiviral therapy is prescribed concomitantly.6 The ACR guidelines encourage the collaboration of rheumatologists with gastroenterologists to monitor patients who receive antiviral therapy. For patients with a history of serious infections, the guidelines recommend using a combination of DMARDs or abatacept over TNF inhibitors.6
In these settings, interest remains in new agents that may provide advantages in efficacy or safety beyond those of currently available RA drugs.
The Role of IL-6 in the Pathogenesis of Rheumatoid Arthritis
RA affects synovial membranes and cartilage, progressively leading to the destruction of articular cartilage and bone erosion.7 The pathogenesis of RA is a multistep progression that involves synovitis, characterized by the infiltration of inflammatory cells, such as macrophages and lymphocytes; synovial hyperplasia with neovascularization; autoantibody production, such as rheumatoid factor and anticitrullinated protein antibody; and excess synovial fluid, ultimately resulting in joint swelling, pain, and stiffness.7,8 During this process, the infiltrating cells and synoviocytes release several proinflammatory cytokines, including TNF-α, IL-1, and IL-6.7,8
Although the etiology of RA is multifactorial, its pathogenesis is strongly associated with the proinflammatory cytokine IL-6.7,8 Serum from patients with RA has been shown to have elevated levels of IL-6, which directly correlates with RA disease severity and radiologic joint damage.9 The overproduction of IL-6 has also been detected in the synovial cells and macrophages in the joints of patients with RA.9
Furthermore, higher levels of soluble IL-6R in synovial fluid were observed in patients with RA versus patients with osteoarthritis, implicating IL-6 as a cause of synovial inflammation.7,10,11 The dysregulated and persistent production of IL-6 plays a key role in the development of many clinical characteristics of RA.7,10,11
IL-6 signaling is induced through classical and trans-signaling pathways, which are implicated in mediating distinct biological effects (Figure).12 In both cases, IL-6 signaling primarily involves 2 molecules, a universal cell-surface signal transducer gp130 that is common to both pathways, and IL-6R, which exists in membrane-bound or soluble forms.12 The transmembrane IL-6R form is expressed predominantly on hepatocytes, neutrophils, monocytes, macrophages, and some lymphocytes, whereas the soluble form of IL-6R is expressed ubiquitously and lacks the transmembrane and cytoplasmic parts of the protein.12
The transmembrane and soluble forms of IL-6R exhibit comparable binding affinity to their ligand IL-6.13 The binding of IL-6 to IL-6R (transmembrane or soluble) on target cells and the recruitment of gp130 triggers the classical signaling pathway, resulting in the stimulation of downstream effector signaling primarily via the Janus kinase and the transactivation of transcription factors, including STAT3, in target cells.7,8,14
The IL-6 trans-signaling pathway is triggered by the binding of IL-6 to soluble IL-6R and subsequent complex formation with gp130.14 In vivo, in contrast to the classical pathway, which is restricted to the few target cells that the membrane-bound IL-6R is expressed on, the trans-signaling IL-6 pathway is stimulated in several types of target cells, which would otherwise be unresponsive to IL-6.14 The IL-6 trans-signaling pathway is strongly implicated in promoting the transition from acute to chronic inflammation, and current evidence supports a proinflammatory role for trans-signaling and a regenerative or an anti-inflammatory role for classical signaling.8,12
IL-6 is a pleotropic cytokine, with several biologic activities, including the regulation of the immune response, inflammation, and hematopoiesis, all of which participate in the articular and systemic symptoms of RA.8 IL-6 is the principal mediator of systemic inflammation–the hallmark of RA–that results in several clinical manifestations, including anemia, osteoporosis, and fatigue.12
The proinflammatory properties of IL-6 include stimulating the production of chemokines and adhesion molecules in lymphocytes, inducing acute-phase proteins through hepatocyte stimulation, and increasing neutrophil counts in the blood.7 Although IL-6 plays a beneficial role during acute inflammation by recruiting neutrophils, it influences the shift to chronic inflammation, favoring monocyte recruitment and leading to macrophage differentiation.12,15
The IL-6 hepcidin axis plays an important role in the symptom manifestation of anemia, the most common systemic RA symptom that results in severe physical disability in many patients with RA.12 IL-6 induces the production of hepcidin, which regulates iron metabolism by preventing iron transport and the release of iron from macrophages.12
In addition, IL-6 stimulates the secretory activity of the hypothalamus-pituitary-adrenal gland axis and increases the production of adrenocorticotropic hormone and cortisol, leading to fatigue in patients with RA.7,12
IL-6 signaling is also implicated in reduced bone formation, defective ossification, and bone destruction and osteoporosis, by inducing osteoclast formation via the upregulation of the receptor activator of the nuclear factor kappa B and its ligand.8,12
Furthermore, neovascularization is purported to support the development and maintenance of the synovial changes observed in RA by facilitating the infiltration of inflammatory cells that trigger synovitis.7 Vascular endothelial growth factor (VEGF), a potent angiogenic factor, plays a key role in IL-6–induced angiogenesis. High levels of VEGF are observed in patients with RA and correlate with greater disease activity, implicating VEGF in the pathogenesis of RA.7 In cultured synovial fibroblasts from patients with RA, IL-6 via the trans-signaling IL-6 pathway resulted in increased VEGF levels.12
The functions of IL-6 in the pathogenesis of RA make it a rational therapeutic intervention in RA. This hypothesis is supported by clinical evidence with the IL-6R inhibitor tocilizumab and the investigational, novel IL-6 blocker sarilumab.9
Sarilumab
Sarilumab is a fully human monoclonal antibody that is directed against IL-6R.5 Sarilumab binds to the transmembrane and soluble forms of IL-6R, and blocks IL-6–mediated cis- and trans-signaling in a dose-dependent manner.16 In addition, sarilumab exhibits a 20-fold greater affinity for human IL-6R than does tocilizumab.5,17
Sarilumab is in advanced clinical trials and a licensing application for its use in the treatment of patients with moderate-to-severe RA is under FDA review, with final FDA approval expected in the second quarter of 2017.
The following discussion describes evidence from clinical trials demonstrating the safety, efficacy, and patient-related outcomes of sarilumab in patients with moderate-to-severe RA.
MOBILITY: Dose-Ranging Phase 2 StudyThe phase 2/3, randomized, double-blind, placebo-controlled, multicenter MOBILITY (Monoclonal Antibody to IL-6Ra in RA Patients: a Pivotal Trial with X-ray) clinical trial evaluated the safety and efficacy of sarilumab in patients with moderate-to-severe RA that persisted despite methotrexate therapy after at least 12 weeks.16
In the phase 2, dose-ranging component of the study, researchers assessed 5 different regimens of subcutaneous sarilumab (100 mg and 150 mg weekly, and 100 mg, 150 mg, and 200 mg every 2 weeks) for 12 weeks. The primary end point was the proportion of patients who achieved improvement of ≥20% per the ACR criteria (ACR20) response rate at week 12.16
Overall, 72% of patients who received sarilumab 150 mg weekly achieved ACR20 compared with 46.2% of patients in the placebo group (multiplicity adjusted P = .0203).16
In addition, 67% of patients who received sarilumab 150 mg every 2 weeks (unadjusted P = .0363) and 65% of patients who received sarilumab 200 mg every 2 weeks (unadjusted P = .0426) achieved ACR20. Based on the overall efficacy, safety, and dosing convenience, sarilumab 150 mg and sarilumab 200 mg every 2 weeks dosing were selected for the phase 3 portion of the MOBILITY study.16
Across dosage groups, the incidence of treatment-emergent adverse events ranged from 43% to 72% with sarilumab versus 47% with placebo. Serious adverse events occurred in ≤6% of patients in any treatment group.16 Overall, 15 (5.5%) patients who received sarilumab had an injection-site reaction compared with 1 (2%) patient who received placebo. Infections were the most common adverse events, but none were serious.16
MOBILITY Phase 3 Study
In the phase 3 portion of the MOBILITY study, the safety and efficacy of 2 regimens (150 mg and 200 mg every 2 weeks) of subcutaneous sarilumab in combination with weekly methotrexate were investigated in patients with active RA despite methotrexate therapy after at least 12 weeks.18
The 3 coprimary end points were the proportion of patients who achieved ACR20 response rate at week 24; the change from baseline in physical function, as assessed by the Health Assessment Questionnaire-Disability Index (HAQ-DI) at week 16; and the change from baseline in the modified Sharp/van der Heijde score of radiographic damage at week 52.18
At week 24, 58% of patients who received sarilumab 150 mg every 2 weeks and 66% of those who received sarilumab 200 mg every 2 weeks achieved ACR20 compared with 33% of patients who received placebo (P <.0001).18
Furthermore, HAQ-DI scores were significantly improved from baseline in patients who received sarilumab 150 mg (–0.53) or sarilumab 200 mg (–0.55) compared with patients who received placebo (–0.29; P <.0001; Table 1).18
At week 52, significant inhibition of structural joint damage was observed with sarilumab 150 mg (0.90) and sarilumab 200 mg (0.25) compared with placebo (2.78; P <.0001).18 Improvements in RA with sarilumab plus methotrexate were maintained for up to 52 weeks and occurred as early as 2 weeks.18
The incidence of treatment-emergent adverse events ranged from 74.5% to 78.1% with sarilumab versus 61.6% with placebo, across dosage groups.18 Infections were the most common adverse events, with serious infections occurring in 2.6%, 4%, and 2.3% of patients who received sarilumab 150 mg, sarilumab 200 mg, or placebo, respectively. Alanine aminotransferase levels increased more than 3-fold the upper limit of normal in 9.5%, 8.0%, and 2.1% of patients who received sarilumab 150 mg, 200 mg, or placebo, respectively, which led to treatment discontinuation in 24 patients.18
Elevations in fasting total cholesterol levels were reported in 36.8%, 43.0%, and 18.3% of patients who received sarilumab 150 mg, sarilumab 200 mg, or placebo, respectively, which led to the initiation of lipid-modifying agents in 29 patients.18 Overall, 8 neoplasms were reported during the study, 4 in the 150-mg sarilumab group, 3 in the 200-mg sarilumab group, and 1 in the placebo group.18
The TARGET Study: Inadequate Response to TNF Inhibition plus MethotrexateThe 3-arm, multicenter, randomized, double-blind, placebo-controlled, phase 3 TARGET clinical trial evaluated the safety and efficacy of sarilumab in patients whose disease did not respond to TNF inhibitor therapy.19 Overall, 546 patients were randomized to receive subcutaneous sarilumab 150 mg, sarilumab 200 mg, or placebo every 2 weeks in combination with background conventional DMARDs for 24 weeks. Patient stratification was based on the number of previous TNF inhibitors and on the geographic region.19
Patients who received sarilumab 150 mg or 200 mg achieved a significantly higher ACR20 response rate at week 24 (55.8% and 60.9%, respectively) compared with placebo (33.7%; P <.0001).19
The coprimary end point of the mean change from baseline in the HAQ-DI score at week 12 was also significantly greater with sarilumab 150 mg (–0.46) and 200 mg (–0.47; P = .0007) versus placebo (–0.26; P = .0004).19
By the end of the study, more patients in the placebo group (34.8%) required rescue treatment compared with patients in the sarilumab 150-mg group (13.8%) and the 200-mg group (14.1%).19 The safety profiles of sarilumab 150 mg and 200 mg were consistent with those reported in the MOBILITY study. Infections were the most common adverse events.19
MONARCH: First-Line Sarilumab versus Adalimumab Phase 3 StudyThe multicenter, randomized, double-blind, double-dummy, phase 3 MONARCH clinical trial evaluated the safety and efficacy of sarilumab versus the TNF inhibitor adalimumab in patients with active RA.20
Patients were randomized to receive subcutaneous sarilumab 200 mg every 2 weeks plus placebo (N = 184) or adalimumab 40 mg every 2 weeks plus placebo (N = 185) for 24 weeks. The primary end point was the change from baseline in the 28-joint disease activity score using erythrocyte sedimentation rate (DAS28-ESR) at week 24.20
The sarilumab 200 mg every 2 weeks group achieved a greater mean change in DAS28-ESR from baseline to week 24 compared with the adalimumab 40 mg every 2 weeks group (−3.28 vs −2.20, respectively; 95% confidence interval [CI], −1.36 to −0.79; P <.0001), establishing its superiority over adalimumab (Table 2).20
Based on the DAS28-ESR scores, the odds of remission were significantly higher in patients who received sarilumab at 12 weeks (odds ratio [OR], 2.61; 95% CI, 1.31-5.20; nominal P = .0051) and at 24 weeks (OR, 4.88; 95% CI, 2.54-9.39; P <.0001).20
In addition, patients who received sarilumab achieved significantly higher ACR20 versus adalimumab (71.7% vs 58.4%, respectively), ACR50 versus adalimumab (45.7% vs 29.7%, respectively), and ACR70 versus adalimumab (23.4% vs 11.9%, respectively; all P≤.0074), and had a significantly greater improvement in HAQ-DI scores (P = .0037).20
Overall, 7.1% of patients who received sarilumab achieved Clinical Disease Activity Index remission at week 24 versus 2.7% of patients who received adalimumab (nominal P = .0468).20 In addition, 41.8% of patients who received sarilumab achieved low disease activity compared with 24.9% of patients who received adalimumab (nominal P = .0005).20
The incidence of treatment-emergent adverse events was 63.6% with adalimumab versus 64% with sarilumab.20 Although the incidence of neutropenia was higher with sarilumab than with adalimumab (13% vs 0.5%, respectively), the infection rate was similar between the 2 agents (28% vs 27%, respectively). Injection-site erythema was more common with sarilumab (7.6%) than with adalimumab (3.3%); headache was more common with adalimumab (6.5%) than with sarilumab (3.8%).20
Patients’ Perspective: Improvement of Functional Disability, Pain, and Fatigue with Sarilumab
Patients with RA experience profound deterioration in health-related quality of life (QOL), and are less able to work outside the home and engage in social activities because of pain, fatigue, and diminished physical functioning.21 Therefore, improving physical functioning and QOL with therapeutic agents are deemed as important and clinically relevant as reducing the signs and symptoms of RA.21
Patient-reported outcomes were tracked in the 52-week phase 3 MOBILITY study to assess the effect of sarilumab in addressing QOL indicators.22 The study evaluated QOL using patient global assessment of disease activity (PtGA), pain visual analog scale, 36-Item Short Form Health Survey (SF-36), the HAQ-DI, and the functional assessment of chronic illness therapy-fatigue (FACIT-F) instruments.22
Patients receiving sarilumab (150 mg or 200 mg) showed clinically meaningful improvements from baseline by week 24 in PtGA, pain, FACIT-F, SF-36, and HAQ-DI compared with patients receiving placebo; these improvements were sustained until week 52.22
Differentiating Sarilumab from Other Biologics in Rheumatoid Arthritis
Several IL-6–targeted biologic drugs for RA are in various stages of clinical development (Table 3).5 Tocilizumab, a recombinant humanized anti–IL-6R monoclonal antibody, is currently the only IL-6–targeting biologic DMARD that is approved by the FDA for the treatment of patients with moderate-to-severe RA and inadequate response to 1 or more DMARDs. Tocilizumab is administered intravenously every 4 weeks, or subcutaneously once every other week, followed by every week dosing, depending on the clinical response.23
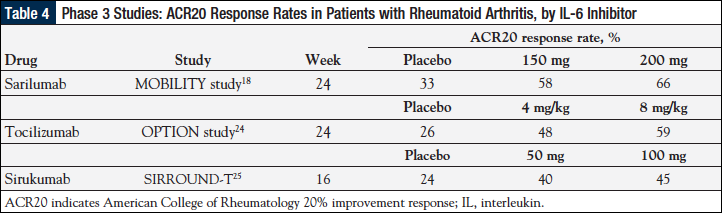
Based on the current evidence, the efficacy and safety data for sarilumab in patients with RA who had an inadequate response to methotrexate are similar to the results seen with tocilizumab (Table 4).18,24,25
However, as previously noted, sarilumab has a 20-fold greater affinity for IL-6 than does tocilizumab,5,17 and in the absence of head-to-head comparative studies, this is expected to translate to greater potency and may also translate to superior efficacy over tocilizumab. Results from future studies will help to define sarilumab’s position among the IL-6 inhibitors. In addition, from a practical standpoint, the use of sarilumab therapy may be more convenient for patients, given its subcutaneous administration every 2 weeks versus weekly dosing with tocilizumab.5
Sarilumab versus TNF InhibitorsTNF inhibitors are often selected to reduce RA symptoms after patients have an inadequate response to conventional DMARDs; however, 20% to 40% of patients do not respond to these treatments and therefore do not gain clinical benefits.9
Until recently, the role of IL-6 inhibitors in the pathobiology of RA was thought to be redundant in patients who did not respond to a TNF inhibitor, because TNF-α acts upstream of IL-6.26 However, the delineation of the IL-6 trans-signaling pathway has significantly altered this notion. With the understanding that TNF-α and IL-6 elicit distinct intracellular signaling and mediate unique functions in RA pathogenesis, switching from a TNF inhibitor to IL-6 inhibitors is considered a rational treatment decision that is supported by the current ACR guidelines.6,26
In light of the recent results from the MONARCH study, which demonstrated the superiority of sarilumab monotherapy over the TNF inhibitor adalimumab, the balance has tilted in favor of the use of IL-6 inhibitors in patients with moderate-to-severe disease activity who are not candidates for methotrexate therapy (because of intolerance or inadequate responses to this agent) and for whom biologic DMARD monotherapy is indicated. Combination therapy with methotrexate may not be appropriate in several clinical settings, including pregnancy, breast-feeding, heavy alcohol consumption, renal or lung disease, or an infection (eg, herpes zoster).
Furthermore, TNF inhibitors are not indicated in certain high-risk patient populations, including patients with CHF and patients with a history of lymphoproliferative malignancies.6 The use of non-TNF biologic DMARDs, such as sarilumab, may fill the treatment gap in these patient populations.
Value-Based Treatment of RA: Cost-Effectiveness Analysis of Treatment Sequence
In the United States, the estimated annual economic burden of RA is equal to €41.6 billion.27 Specialty drugs alone account for at least 50% of the cost of care associated with RA.28 In addition to direct costs from drugs, clinic visits, and hospitalization, RA is associated with considerable indirect costs, including reduced productivity and lack of employment opportunities.27,28
Although the benefit of using an effective biologic DMARD with a favorable safety profile to halt joint destruction is undisputed, given the wide array of RA drugs, and the high costs of biologic agents, economic evaluation of these agents is critical to inform the optimal treatment sequence and healthcare resource utilization.29,30 The treatment goal for all patients with RA is to achieve remission, or at least low disease activity, using the most cost-effective therapy possible.29
In a recent systematic international review, Joensuu and colleagues examined the cost-effectiveness of biologics for RA.30 Based on direct costs alone, the incremental cost-effectiveness ratio (ICER) of the TNF inhibitors ranged from €39,000 to €1,273,000 per quality-adjusted life-years (QALYs) compared with conventional DMARDs in patients who have not received conventional DMARDs.30 ICER describes the ratio of the additional costs of a treatment (vs an alternative treatment) to QALYs gained.30
However, among patients with an inadequate response to conventional DMARDs (based on direct costs alone), biologic DMARDs were associated with ICERs ranging from €12,000 to €708,000 per QALY. None of the biologic agents were reported to be more cost-effective than the others.30
The willingness-to-pay threshold in this cost-effectiveness analysis was €35,000 per QALY, based on which biologic DMARDs were not cost-effective in patients who had not received conventional DMARDs, supporting the current recommendation of initiating treatment with conventional DMARDs.30
With willingness-to-pay thresholds of €50,000 to €100,000 per QALY, biologic DMARDs may be cost-effective among patients with an inadequate response to conventional DMARDs. Among patients with an inadequate response to TNF inhibitors, ICERs for the second-line biologic DMARDs ranged from €26,000 to €1,347,000 per QALY compared with conventional DMARDs.30
Based on these cost-effectiveness models and the ACR guidelines, conventional DMARDs are cost-effective at disease onset, and therapeutic escalation with TNF inhibitors is cost-effective when conventional DMARDs fail; in the case of a lack of response to TNF inhibitors, rituximab and abatacept may be the most cost-effective treatment options.29,30
Overall, country-specific differences make cost-effectiveness assessments challenging; differences arising from drugs being in the market for different time periods were noted in this analysis, as well as issues relating to insufficient reporting of data sources and problematic methodologic details.30
Conclusions
Despite treatment advances attained with biologic DMARDs for RA, disease progression often persists in patients who have an inadequate response to or are intolerant of these agents, or because of contraindications related to specific biologic DMARDs, underscoring the need for more effective therapies.
With the understanding that IL-6 is the chief proinflammatory cytokine associated with chronic inflammation in RA, IL-6 blockers, such as sarilumab, offer a promising treatment option for patients with moderate-to-severe RA; this is especially true in patients who do not respond to TNF inhibitor therapy; in cases when TNF inhibitor therapy is contraindicated; and, based on the data from the MONARCH study, when the IL-6 blocker is used as front-line therapy in place of TNF inhibitor therapy.
Sarilumab is currently being reviewed by the FDA, and encouraging data from clinical trials indicate that this new IL-6 blocker may be an important addition to the RA treatment armamentarium. The challenge for rheumatologists and for payers will be to find the appropriate niche for this agent within an optimal sequencing approach with the currently available biologic DMARDs for the treatment of patients with moderate-to-severe RA.
References
- Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2010;2:247-256.
- Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. 2014;20(7 suppl):S128-S135.
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023-2038. Erratum in: Lancet. 2016;388:1984.
- Curtis JR, Zhang J, Xie F, et al. Use of oral and subcutaneous methotrexate in rheumatoid arthritis patients in the United States. Arthritis Care Res (Hoboken). 2014;66:1604-1611.
- June RR, Olsen NJ. Room for more IL-6 blockade? Sarilumab for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2016;16:1303-1309.
- Singh JA, Saag KG, Bridges SL Jr, et al; for the American College of Rheumatology. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68:1-25.
- Hashizume M, Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis. 2011;2011:765624.
- Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. Biomed Res Int. 2014;2014:698313.
- Kim GW, Lee NR, Pi RH, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res. 2015;38:575-584.
- Matsumoto T, Tsurumoto T, Shindo H. Interleukin-6 levels in synovial fluids of patients with rheumatoid arthritis correlated with the infiltration of inflammatory cells in synovial membrane. Rheumatol Int. 2006;26:1096-1100.
- Narazaki M, Tanaka T, Kishimoto T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert Rev Clin Immunol. 2017 Mar 3. Epub ahead of print.
- Dayer JM, Choy E. Therapeutic targets in rheumatoid arthritis: the interleukin-6 receptor. Rheumatology (Oxford). 2010;49:15-24.
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237-1247.
- Scheller L, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321-329.
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(suppl 2):S3.
- Huizinga TW, Fleischmann RM, Jasson M, et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73:1626-1634.
- Rafique A, Martin J, Blome M, et al. Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human il-6 receptor (il-6r) alpha. Ann Rheum Dis. 2013;72(suppl 3):A797.
- Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67:1424-1437.
- Fleischmann R, van Adelsberg J, Lin Y, et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2017;69:277-290.
- Burmester GR, Lin Y, Patel R, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76:840-847.
- Strand V, Wright GC, Bergman MJ, et al. Patient expectations and perceptions of goal-setting strategies for disease management in rheumatoid arthritis. J Rheumatol. 2015;42:2046-2054.
- Strand V, Kosinski M, Chen CI, et al. Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial. Arthritis Res Ther. 2016;18:198.
- Actemra (tocilizumab) injection [prescribing information]. South San Francisco, CA: Genentech; 2010.
- Smolen JS, Beaulieu A, Rubbert-Roth A, et al; for the OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987-997.
- Aletaha D, Bingham CO III, Tanaka Y, et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet. 2017;389:1206-1217.
- Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013;9:154-163.
- Taylor PC, Moore A, Vasilescu R, et al. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36:685-695.
- Gleason PP, Alexander GC, Starner CI, et al. Health plan utilization and costs of specialty drugs within 4 chronic conditions. J Manag Care Pharm. 2013;19:542-548.
- Benucci M, Saviola G, Manfredi M, et al. Cost effectiveness analysis of disease-modifying antirheumatic drugs in rheumatoid arthritis. A systematic review literature. Int J Rheumatol. 2011;2011:845496.
- Joensuu JT, Huoponen S, Aaltonen KJ, et al. The cost-effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review. PLoS One. 2015;10:e0119683.
MOA Magnifier™ is published by The Lynx Group, LLC, 1249 South River Rd, Suite 202A, Cranbury, NJ 08512. Copyright © 2018 by The Lynx Group, LLC. All rights reserved. MOA Magnifier™ is a registered trademark of The Lynx Group, LLC. No part of this publication may be reproduced or transmitted in any form or by any means now or hereafter known, electronic or mechanical, including photocopy, recording, or any informational storage and retrieval system, without written permission from the Publisher. Printed in the United States of America.
The ideas and opinions expressed in this issue do not necessarily reflect those of the Editorial Board, the Editors, or the Publisher. Publication of an advertisement or other product mentioned in this issue should not be construed as an endorsement of the product or the manufacturer’s claims. Readers are encouraged to contact the manufacturers about any features or limitations of products mentioned. Neither the Editors nor the Publisher assume any responsibility for any injury and/or damage to persons or property arising out of or related to any use of the material mentioned in this publication.
EDITORIAL CORRESPONDENCE should be addressed to EDITORIAL DIRECTOR, The Lynx Group, LLC, 1249 South River Rd, Suite 202A, Cranbury, NJ 08512. Phone: 732-992-1880. Correspondence regarding permission to reprint all or part of the article published in this supplement should be addressed to REPRINT PERMISSIONS DEPARTMENT, The Lynx Group, LLC, 1249 South River Rd, Suite 202A, Cranbury, NJ 08512.